
Water vapor is a substance in the gas phase that is at a temperature lower than the critical point. Due to this characteristic, vapor can be condensed into a liquid or solid by increasing its pressure without reducing its temperature.
That is, it is a gas that can be condensed at a constant temperature by increasing the pressure. On the other hand, to convert a non-vaporous gas into a liquid, it is not enough to increase the pressure; the temperature must be decreased.
For example, water has a critical temperature of 374°C (647 kelvin), which is the highest temperature at which liquid water can exist.
Vapor can coexist with a liquid or solid. In this case, the two phases will be in equilibrium, and the gas pressure will be equal to the equilibrium vapor pressure of the liquid (or solid).
Normally, the word vapor, if the substance is not specified, usually refers to water vapor.
Definition: What is water vapor?
Water vapor is a gas produced by boiling when water is heated to 100°C (the boiling point of water) at one atmosphere of pressure.
At this point, the water is said to be in a phase change. At this point, if we continue to supply heat, the temperature will not increase until the water has evaporated.
Under these pressure conditions, above 100 degrees Celsius, pure water is in the gas state.
We often use this term to refer to wet vapor, the aerosol of water droplets that form when they condense, fog, etc. However, dry vapor is invisible.
Relative humidity is the ratio of the partial pressure of water vapor in a gas (mainly in air) to the equilibrium pressure of saturated vapor at a given temperature.
Absolute humidity is the amount of water vapor contained in 1 cubic meter of air at a given temperature.
Vapor state
The vapor state is the state in which a gas is found when it is below the critical temperature.
In the gas state, the molecules that make it up do not react with each other by forming molecular bonds but rather tend to repel each other. By repelling each other, the water molecules adopt the shape and volume of the container that contains them and tend to separate and occupy all the available volume.
Characteristics of water vapor
Water vapor is the gaseous phase of water and has a series of physical and chemical properties that make it essential for multiple natural and industrial processes.
1. Colorless and odorless
Water vapor in its pure state is a colorless and odorless gas. However, when it is present in large quantities and begins to condense, it can become visible in the form of fog or clouds of small water droplets suspended in the air.
2. Part of the hydrological cycle
It is an essential component of the water cycle. It is produced by evaporation and transpiration, rises into the atmosphere, condenses in the form of clouds, and finally returns to the Earth's surface in the form of precipitation (rain, snow, or hail).
3. Greenhouse gas
Water vapor acts as a greenhouse gas, absorbing and retaining heat in the Earth's atmosphere. It regulates the planet's temperature and plays a key role in climate and meteorology.
4. Expansion and pressure
When water turns into steam, it experiences a large expansion in volume. At high temperatures and pressures, this phenomenon is used in engines and turbines to generate movement and mechanical energy.
5. Thermal conductivity and heat capacity
Water vapor has a high heat capacity, which means it can store and transfer large amounts of heat. For this reason, it is used in heating systems, energy production, and industrial processes where heat transmission is required.
6. State changes and condensation
When cooled, water vapour condenses into small liquid droplets, forming clouds, fog or dew. This process is key to the formation of precipitation and the regulation of the thermal balance of the atmosphere.
Difference between gas and steam
Steam is a type of gas, but not all gases are steam.
If we compress a gas while maintaining the temperature, the gas does not change state, it remains a gas. However, by increasing the pressure at a constant temperature, the vapor can become liquid.
Gas occupies all available space, while vapor does not behave this way.
Importance of steam
Steam is an essential element for life on Earth, playing a fundamental role in various natural and technological processes.
Its presence in the atmosphere influences the climate and the hydrological cycle, while its use by humans has been key to the development of industry and energy production. From the Industrial Revolution to the present day, the use of steam has driven significant advances in the generation of mechanical work and electricity.
Importance in the environment
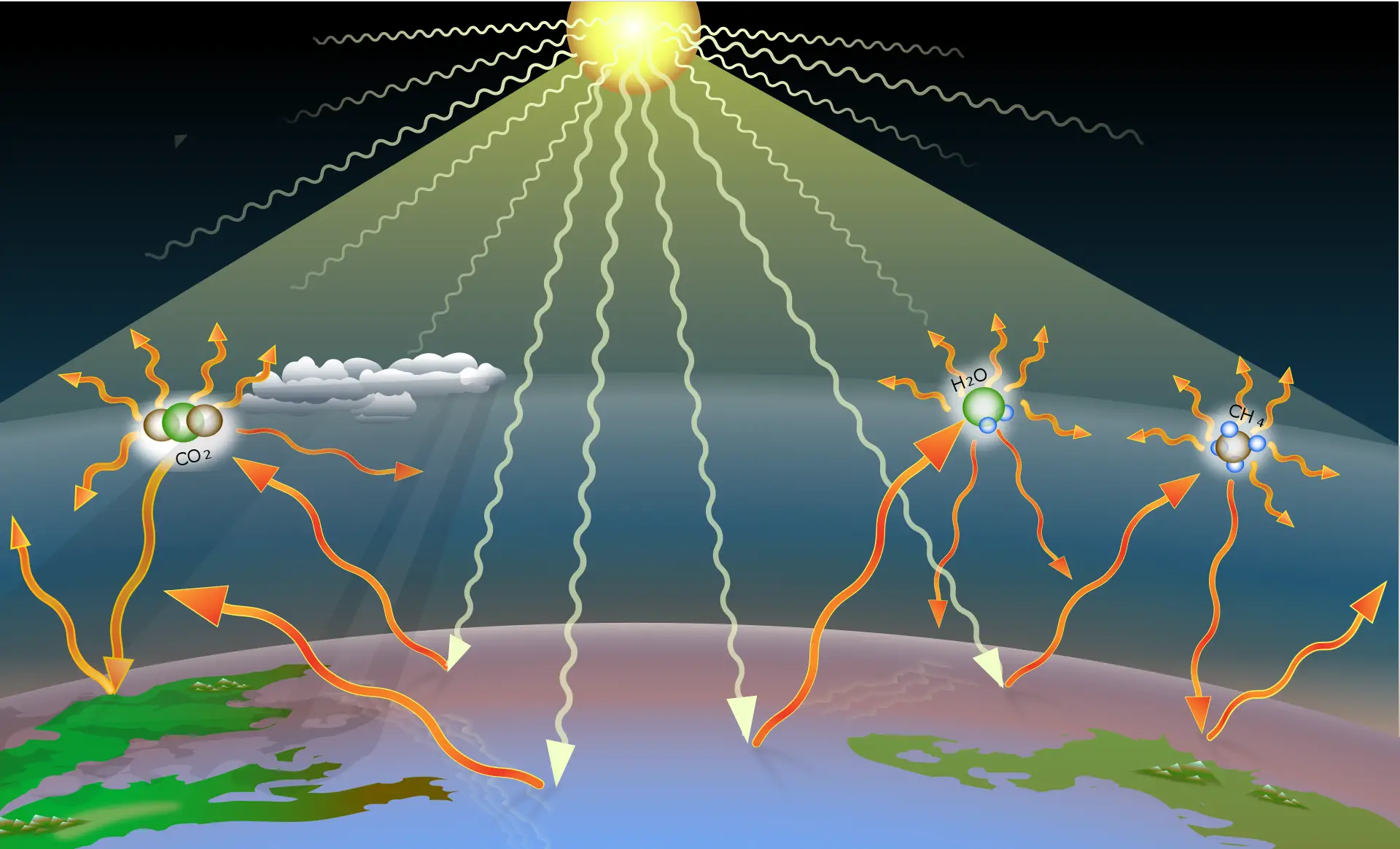 Water vapor is a crucial component of the Earth's atmosphere and a greenhouse gas that, like carbon dioxide, contributes to the planet's thermal regulation. It absorbs and retains part of the solar radiation reflected by the Earth's surface, helping to maintain a temperature suitable for life. However, changes in its concentration can influence global warming and climate variability.
Water vapor is a crucial component of the Earth's atmosphere and a greenhouse gas that, like carbon dioxide, contributes to the planet's thermal regulation. It absorbs and retains part of the solar radiation reflected by the Earth's surface, helping to maintain a temperature suitable for life. However, changes in its concentration can influence global warming and climate variability.
In addition, water vapor plays an essential role in the hydrologic cycle, which is the continuous process of water circulation on Earth. Through evaporation, water passes from rivers, oceans, and lakes into the atmosphere, forming clouds that subsequently produce precipitation.
This cycle is vital for maintaining ecosystems, climate, and providing freshwater for human consumption, agriculture, and other essential activities.
Importance in the industry
The properties of steam enabled the development of the steam engine, an invention that completely transformed industry and society during the Industrial Revolution.
Thanks to steam's ability to generate movement, steam engines were implemented in factories, facilitating the mechanization of production and increasing the efficiency of industrial processes. This marked the beginning of an era of modernization, with advances in sectors such as mining, manufacturing, and transportation.
In the field of transport, steam locomotives revolutionized land mobility, allowing for faster and more efficient travel. Similarly, steamships facilitated the expansion of maritime trade, reducing sailing times and increasing cargo capacity.
These advances contributed to market integration and global economic growth.
Electricity generation
Steam continues to play a crucial role in electricity production, especially in thermal and nuclear power plants.
In these facilities, thermal energy obtained from fossil fuels (such as coal, gas, and oil) or from nuclear fission is used to heat water and generate high-pressure steam. This steam drives steam turbines connected to electric generators, transforming thermal energy into mechanical energy and, subsequently, into electricity.
This operating principle is the basis of many power generation plants around the world, as steam makes it possible to harness different heat sources to efficiently produce electricity.
In addition, steam turbines have evolved with technological improvements that increase their efficiency and reduce their environmental impact, contributing to the transition towards a more sustainable energy system.