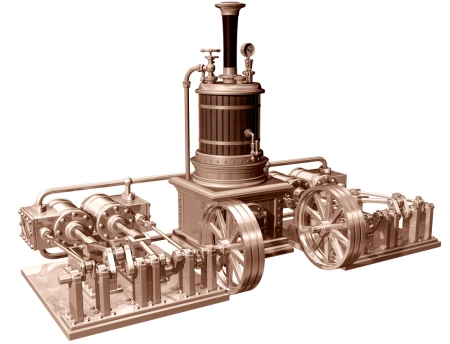
A steam engine is an external combustion engine that converts thermal energy into mechanical energy by using high-pressure steam. Its operation is based on heating water to generate steam, whose expansion drives a mechanical mechanism, such as pistons or turbines.
The heat needed to generate steam comes primarily from the combustion of coal, although it can also be obtained from wood, oil, nuclear reactions or even solar energy.
Considered one of the most revolutionary inventions in history, the steam engine played a key role in the Industrial Revolution, driving the development of transportation, industry and power generation during the 19th century.
Define Steam Engine
A steam engine is a heat engine that converts thermal energy from steam into mechanical work. It operates by heating water in a boiler to generate steam, which expands and creates pressure. This pressurized steam moves a piston in a reciprocating engine or spins a turbine in a steam turbine, producing rotational motion.
The process relies on external combustion, where fuel—such as coal, wood, or oil—is burned outside the engine to heat the water.
How did steam engines work
The operation of a steam engine follows these basic steps:
- Heat generation: A fuel (coal, wood or oil) is burned to heat water in a boiler.
- Steam production: Water reaches its boiling point and turns into high-pressure steam.
- Energy conversion: Steam pressure is used to move a mechanical mechanism, such as a piston or turbine.
Steam generator or boiler
The steam generator, or boiler, is an essential part of the system. Here, water is heated into steam, which is then directed to the engine. Depending on the design, the steam engine engine can be of two main types:
- Reciprocating engine: Uses a piston that moves back and forth.
- Rotary engine: Drives a steam turbine to generate continuous motion.
Reciprocating engine of a steam engine
In a reciprocating engine, steam drives control valves that allow a piston to move in both directions. In each cycle, the engine goes through two active phases, unlike the four-stroke internal combustion engine, where only one expansion phase occurs every four cycles.
Since the second half of the 19th century, most steam engines adopted double, triple or even quadruple expansion systems, using multiple cylinders in series to improve efficiency and make better use of steam energy.
Steam turbines
Steam turbines almost completely replaced reciprocating engines in the maritime field and later in electricity generation. They were eventually replaced in many applications by internal combustion engines and gas turbines, although they remain essential in power plants, where they are used to drive three-phase alternators and generate electricity.
Caractheristics of the steam engine
The main features of the steam engine are:
1. Conversion of thermal energy into mechanical energy
The steam engine transforms heat into mechanical work. When water is heated and steam is generated, it expands and exerts pressure on a piston or turbine, creating useful movement.
2. Use of a thermodynamic cycle
It works by means of a cycle of expansion and contraction of steam, generally following the Rankine cycle. This process allows the continuous generation of mechanical energy from heat.
3. Steam pressure as a driving force
The steam generated reaches high pressures that drive the machine's mechanism. The amount of pressure determines the power and efficiency of the system.
4. Steam flow regulation
The system has valves that control the entry and exit of steam, allowing the speed, power and direction of movement to be adjusted as needed.
5. Reuse of steam in some models
In some more advanced versions, the steam is condensed and reused to improve efficiency and reduce fuel and water consumption.
6. Versatility of application
Steam engines were used in many areas, such as transport (trains and ships), industry (factories and mines) and electricity generation, revolutionising production and mobility.
7. Variable efficiency depending on the design
Efficiency depends on the type of machine, the fuel used and the use of steam. Early versions were underperforming, but improvements such as Watt's machine increased their efficiency.
8. Dependence on external fuel
To operate, the steam engine requires an external heat source, such as coal, wood or oil, which influences its operating cost and sustainability.
Who invented the steam engine?
The development of the steam engine was not the work of a single inventor, but the result of a series of advances over more than a century.
Thomas Savery (1698): the first steam pump
The first working steam device was created by Thomas Savery in 1698. It was a water pump that used condensing steam to create a vacuum and lift water from deep wells. Although useful for pumping in mines and water stations, its design was limited and it could not generate continuous mechanical power.
Thomas Newcomen (1712): the atmospheric engine
In 1712, Thomas Newcomen improved Savery's pump by incorporating a piston and cylinder, inspired by the ideas of the French scientist Denis Papin. His atmospheric engine allowed for more efficient and continuous operation, becoming the first commercially viable steam engine. It was mainly used in coal mines to extract water.
James Watt (1769): the modern steam engine
The great leap forward in steam technology came in 1769 with James Watt, who perfected Newcomen's design. His innovations made the steam engine much more efficient and practical for industrial use and transportation.
James Watt's Innovations
- Separate condensation chamber: Prevented heat loss by keeping the cylinder at a constant temperature.
- Using steam pressure instead of atmospheric pressure: Improved engine efficiency and power.
- Conversion of linear to circular motion: Made possible its application in industrial machinery and locomotives.
- Automatic regulators: Mechanisms such as the butterfly valve allowed the speed of the machine to be controlled.
- Lower fuel consumption: Reduced operating costs compared to Newcomen machines.
In recognition of his work, the unit of electrical power in the International System of Units bears his name: the watt (W).
Impact of the steam engine
Watt's engine revolutionised industry, marking the beginning of the Industrial Revolution. His company, Boulton & Watt, played a key role in the commercialisation of these machines, applying them in factories, railways and steamships.
Denis Papin: a forgotten precursor
French scientist Denis Papin had been experimenting with steam long before Watt. In 1679, he designed a sealed boiler with a piston that rose with heat and fell with the condensation of steam. He also introduced a safety valve system, essential in the development of modern boilers.
Although his design was not commercially applied in his time, it laid the groundwork for later advances in steam technology.